BLOG POSTS
.png)
Radial ArteryWhat is Radial Artery?All arteries have a pulse, but it's easier to palpate (feel) the pulse or heartbeat at certain locations. it's easier to feel the pulse or heart beat when the artery is near the surface of the skin and when there's firm tissue (such as a bone) beneath the artery. The three c... Read More |
Nurses warn of worse staffing levels and rise in stress amid Covid-19Worsened staffing levels, increased stress and growing concerns for the wellbeing of colleagues are just some of the issues reported by nurses working during the pandemic, a new survey has found.The latest Royal College of Nursing survey has explored the impact of the coronavirus pandemic on the... Read More |

Action needed to support retention of nursing staff in WalesWales staffing act must be extended to all settings where NHS Wales commissions or provides nursing care. National action across Wales needs to be taken to support the retention of nursing staff, so safe nurse staffing levels can be achieved, the RCN has said.The comments were made as RCN W... Read More |

Nursing staff stretched to breaking point over workloadsFindings from the RCN’s recent employment survey show that nursing staff across the UK are under such pressure that six out of 10 say they cannot provide the level of care they want to.The annual survey, which was first carried out in 1986, also shows that barely a quarter of respondents think th... Read More |
Chip Device Heals Injuries In SecondsPicture this - you suffer a car accident and your leg is broken. Within moments a small chip-like silicone device is placed on the broken leg - it reprograms the skin cells beneath and treats the injury - in a matter of seconds. Sounds like the stuff of science fiction, right? Nope. This is... Read More |

Unison nurses will ‘work to rule’ in Northern Ireland pay rowNurses in Northern Ireland will refuse to interrupt their breaks or attend meetings with managers over a period of more than three weeks in a protest over pay and staffing levels.The union Unison has announced further details about its plans for strike and industrial action among its health and... Read More |

First survey by new data unit shows district nurses stretched to limitsAlmost half of UK district nurses are planning to leave the profession within the next six years, according to the first major report produced by a new community nursing data hub.The research was carried out by the Queen’s Nursing Institute’s new International Community Nursing Observatory (ICNO... Read More |

Nurse launches podcast to dispel fears about psychiatric hospitalsA newly qualified mental health nurse has given patients detained in psychiatric hospital a platform to share their experiences in a bid to challenge fears and misunderstanding amongst the public.Mr Waldron told, he felt it was important to ensure the voice of patients were heard because th... Read More |
Certain professions and late dinners bad for women’s heart healthA new study from a team from Dornsife School of Public Health at Drexel University in Philadelphia, Pennsylvania, has revealed that post-menopausal women in certain professions are more likely to suffer from heart diseases and other heart problems. Many of these jobs include those in health care... Read More |
.jpg)
Stress and disrupted circadian rhythm affect metabolismThe researchers studied mice models in the laboratory wherein they introduced genetic alterations in various parts of the circadian rhythm machinery. They also exposed the mice to social stress, wherein they exposed the male mice to an unknown and dominant male. The male mice had increased stres... Read More |
.jpg)
How to Become a Family Nurse Practitioner OnlineIf you are a licensed registered nurse you may well be able to enroll in a new online Master of Science in Nursing degree program with a strong focus in Family Nurse Practitioner. This program, offered by Herzing University Online, is available in 27 states in the USA. The University... Read More |

CE series focuses on essentials of speaking Spanish for nursesBenefits of Spanish for nurses and other prosLearning at least basic Spanish, according to Long, can greatly help RNs provide good care.Long teaches our Focused CE series Basic Spanish for Healthcare Providers, along with Speedy Spanish for Healthcare Providers, a 0.5-hour cour... Read More |
More nurses and midwives leaving than joining, new figures showThe number of nurses and midwives leaving the profession has risen 51 percent in just four years, with those under the age of retirement citing low pay and poor working conditions.New figures from the Nursing and Midwifery Council (NMC) show that for the first time in recent history m... Read More |
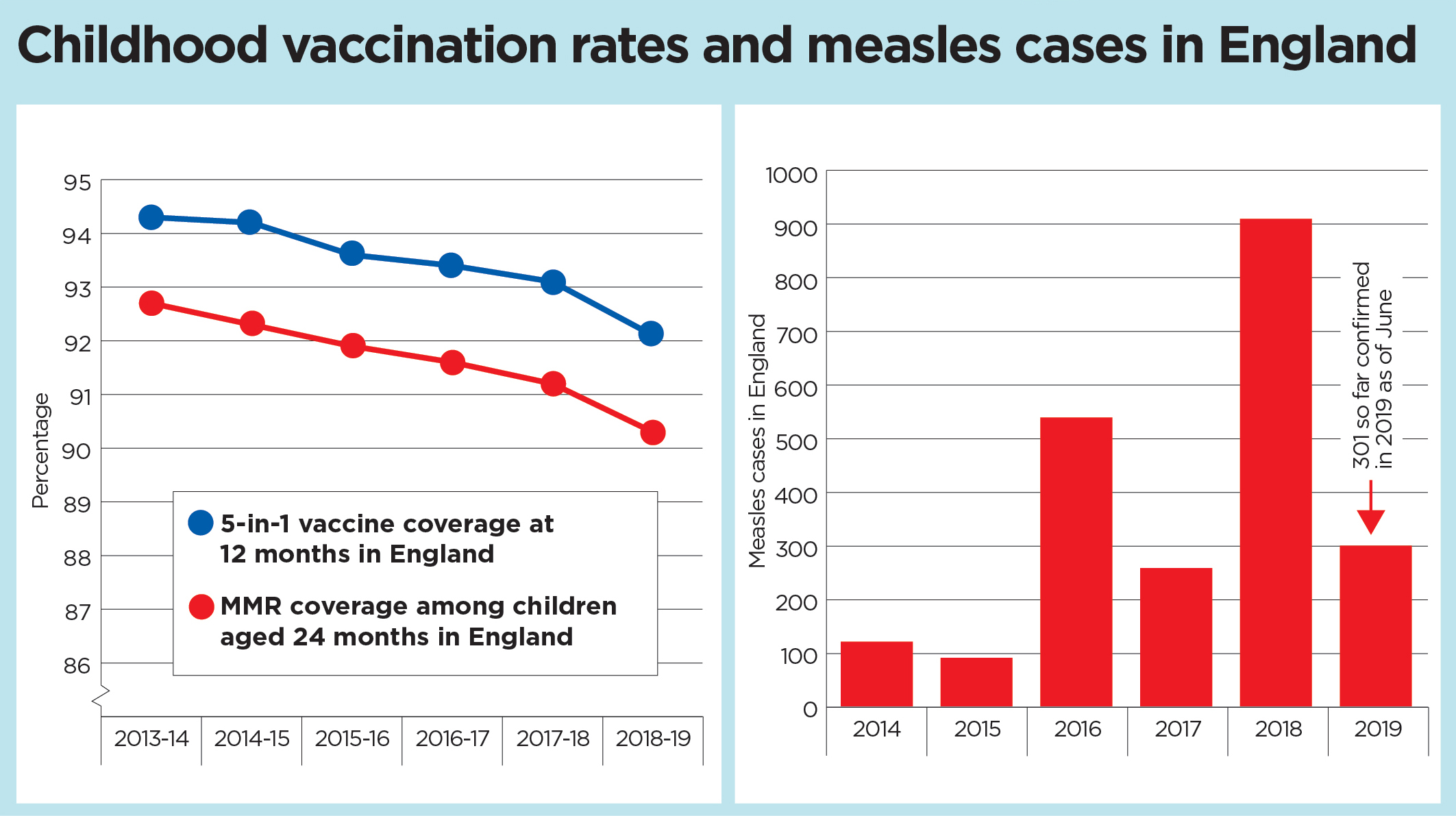
Analysis: What are the key reasons behind the fall in childhood immunisation ratesThe blame has been often pushed onto so-called anti-vaxxers and anti-vaccination messages, but those among the profession and beyond believe bigger problems lie with workforce challenges and the healthcare system itself. Helen Bedford, professor of children’s health and a nurse by background, sa... Read More |
Do you think people in comas can hearYes. I, too, had a tough time accepting that comatose patients can hear us because, like many others, I was fooled by movies into believing that coma = a state of being sort of dead.But then, in the first year, just before allowing us to start clinical rotations at the hospital, the school gave... Read More |
As a surgical nurse, what's the weirdest thing you've seenIt’s difficult to say which is the weirdest as you see a lot of odd things! There was a Vicar with a potato lodged in his bottom, a teenage boy with a glass tomato sauce bottle stuck on his penis (which he had tried to smash off with a hammer), an elderly gentleman with an umbrella handle stuck... Read More |

Can a midwife become a gynecologistYou would need to apply to medical school then to an Ob-gyn residency. I have met a few nurses that have done just that, RN to MD but not from CNM to MD.But if you want to do surgery than that is the way to go.The midwives that were in my practice did a lot of office gyn (Well Woman Care) but h... Read More |
What are some interesting country specific facts about plastic surgeryIran isn’t exactly known as a beacon of open societies.Islamic law is strictly enforced in the country. We (Westerners) often read up on and then practice great caution when traveling to such regions as it is, culturally, a world away from the life we live here.They used to legislate what makeu... Read More |

Do mental health nurses wear uniforms in the US. Why!Mental health workers are encouraged to dress in street clothes. Mental health problems have such a stigma that people fear being observed talking to a mental health worker for fear of repurccusions, Also, patients tend to have bad associations with scrub wearing professionals, it could trigg... Read More |
What is the difference between ICU and CCU in the hospitalICU is the Intensive Care Unit. It is where the sickest or most injured patients are kept. Our hospital had an ICU staff ratio of 1 patient/1 nurse. It is equipped with almost every item to sustain a life within easy reach and has separate care practices per case. It also has a stricter set of r... Read More |

Do you trust foreign-educated health care providers as much as their US-graduate counterpartsI suppose, the author of the question means that foreign-trained doctors conduct themselves less professionally comparing to their American-born counterparts.Here is my observation, as I am a foreign-educated doctor myself: No foreign doctor (except Canadian) can practice medicine in USA indepen... Read More |
How are male nurses viewed by women in the health fieldI am a male nurse here. The nursing field is traditionally and still remains a female-dominated field. For me, I have no problems with it. My colleagues and I are more concerned about the heavy workload to be done in so little time than bickering on gender-related issues. If you are in the ward,... Read More |
What are the emerging challenges in pediatric nursingStaffing is always a problem everywhere. Cost effective plans to keep the hospital in the black, makes things difficult in all departments. Visitor policies can change back & forth. Type of new care for conditions will be a challenge! Keeping toddlers in beds or cribs is a constant challenge... Read More |

Why do most Americans leave their old family members in nursing homes or just avoid their parents in generalMost Americans don’t. It’s too expensive. There are a few reasons why it becomes necessary:· Most older people have very fixed opinions about how things should be that they first formed in their youth when the world was different. Hey, the world ha... Read More |
Are dental implants impossible for women who have had bone loss in their jaws due to the use of meds for osteoporosis and the treatment of estrogen receptor-positive breast cancerDental implants are an elective treatment modality. As in, they are not urgently and compulsarily required. There are substitutes available for them.Dental implant surgery requires an efficient immune system, coagulative system, and reparative system. There are myriad of factors, which can disru... Read More |

What is the most stressful part about being an oncology nurseIn my opinion the most stressful thing about being an oncology nurse is finding out that patients are terminal with no chance of recovery. It's very sad especially when you have to be in the room to break the news or list their options knowing there is no quality of life and they will likely be... Read More |

Is it wrong that I hate nursing schools and wish nurses/nursing lecturers rotted in hell? What can I do to save my soul after a "nursing" disasterIn addition to the other responses here, I'd like to add a story of mine:I was part of a class that was accused of cheating (except for the one who told the administration). A fire alarm was pulled by a middle schooler that was on a field trip during our pharmacology final, and we had to give ou... Read More |

Do you have any regrets for putting your parents in a nursing homeIn a way I do, at least in the beginning. I was her beneficiary and I knew that if she stayed at home all her money would go to her at-home care, and when that ran out, my husband and I would be footing the bill as we already were for his own mother. It would be putting us in a deep financial ho... Read More |
Do doctors ever do surgery on themselvesA nurse here.I gave myself a flu shot. I didn’t want to go to the CVS and pay $20 for it. We had gallons of it for the patients and I wanted to get one. That day I knew I couldn’t be a drug user (or a diabetic who had to give himself insulin!)I once tried to draw my own blood. That was a disaste... Read More |

What is the most important part of being a good surgical nurseFirst and foremost, we need to dissect what makes a “good” Nurse. I would say the key elements are having an empathetic, caring, and warm nature. You need to feel comfortable being around sick people and staying calm in stressful situations. You must be genuine and honest at all times, making sur... Read More |
How many times do hospital nurses have to wash their hands and/or replace their glovesIt depends on how many patients they care for. They should be washing their hands with each patient contact and before they glove and after they glove also they need to wash them well after they use the BR. themselves. If the person has infectious stool or drainage you also would not want to mov... Read More |
Why don't dental nurses ever speak to patientsIt may be the strategy of the individual dentist. Staff in my practice converse w/ patients to put them at ease and because the assistant needs patient cooperation for some procedures the staff does. The more the staff can do the easier it is on the dentist. At the same time this can be risky be... Read More |
Do mental health nurses ever give needles to patientsWe don’t call them “needles.” We call them syringes. In 25+ years of psych nursing, I would estimate that I’ve given approximately 10,000 IM injections.The number is so large not because I’m a crazy Nurse Ratched as so many would like to believe, but because psych patients get sick, just as non-... Read More |
How much does diet affect mental healthI would say - the diet affects mental health directly. In fact, the most efficient protocols for dealing with mental health and neurological issues (autism, epilepsy, etc.) involve radical (to some) changes in diet (for autism, for instance, high-fat and low-carb ketogenic diets prove very effec... Read More |
What makes a great paediatricianI have been a nurse for 9 years.8 of those in pediatrics, with the most recent in pediatric critical care. I work with COUNTLESS resident physicians and we receive a new batch of them every June. To say I am biased would be fair.I have my favorite doctors. I can honestly say it’s not fair to judge... Read More |

Can powder formula be given to babies under 2 monthsYes, most formulas are… formulated for infants from new-born to 12 months of age.I would pay attention to its designation as an infant formula. You don’t want to give toddler formula to a new-born… although in a pinch it may still work… on occasion. The formula market has become now so s... Read More |
What kind of nurses take care of new born babiesThere are generally three types of nurses that care for new born in the hospital setting: Labour and Delivery Nurses typically assist with deliveries and care for the baby immediately following birth (taking vital signs, cutting the cord, and assessing the APGAR score). Maternity Nur... Read More |
Which is The Best Home Nursing Service For The Care Of The Elderly Imagine this situation. Hands always search for a hold, whatever eyes see is blurred and a little walk within the house, the knees are in pain! This is how elderly age feels.At this tenacious and delicate phase of life, frequent visit to the hospitals is the most challenging and tiresome... Read More |
As a nurse, have you ever gone against a doctor’s diagnosis and been rightYes I have. This happened in an Army Medical Center when I was an Army nurse. They had resident physicians there that had graduated from med school and who were making their rotations through different patient care areas of the hospital.I had a patient who was having cardiac arrhythmias due to t... Read More |

How much do surgeons earn in India(specialty vs salary)The average salary for a General Surgeon is Rs 1,166,240 per year. People in this job generally don't have more than 20 years' experience. Experience strongly influences pay for this job.Pay Experience for a General Surgeon has a positive trend. An entry-level General Surgeon with less than 5 ye... Read More |

As a nurse, what is the most shocking statement you ever heard from a young childIm not a nurse but a doctor. Well I remember the 12-year-old boy we had on the paediatric floor with a fractured shaft of femur. I was told by my boss to give him one or another antibiotic by I/V push. When I entered his room, he looked me up and down and then said “You, you’re that f*****g fat,... Read More |

As a nurse, have you ever cried over a patientMy wife's story: I worked in Infectious Disease in the ’80 s when AIDS was little understood beyond being a death sentence. My patients varied from a very young hemophiliac man, to a mother of 3 infected by her husband, who promptly left her, to IV drug users, to the largest category, gay men. T... Read More |
Are we finally going to have a vaccine for Alzheimer'sDementia has skyrocketed to become the fifth biggest cause of death worldwide, Alzheimer’s constitutes about 70 per cent of these cases. Alzheimer's results in progressive loss of memory and cognitive function and is devastating both to those who have it and to their loved ones.Researchers have... Read More |
The Role of Nurses in a Nursing HomeThere are basically three types of nurses in a nursing facility: Registered Nurse (RN), Licensed Practical Nurse (LPN), and Certified Nursing Assistant (CNA), and sometimes a Nurse Practioner (NP). Also, an RN who takes specialized graduate courses in geriatric care and obtains a certificate or... Read More |
What’s a time when you had a serious problem but a doctor outright refused to help youI was 48 years old and I got deathly ill at work one day. Couldn’t hold my head up or stay awake. Went home that afternoon and slept to the next morning. The next day I treated myself like I had a virus but by the evening I couldn’t stand it anymore and went to minor med. I insisted I had strep... Read More |

What Things Frustrate Nursing Staff And Doctors The Most1. Please keep a list of the medications you're taking along with the doses, and if you're unclear on your own medical history, add why you're taking them too! This is a significant issue with the older populations who are more likely to be on many different medications, but it happens with peop... Read More |

How Are Male Nurses Viewed By Women In The Health FieldI am a male nurse here. The nursing field is traditionally and still remains a female-dominated field. For me, I have no problems with it. My colleagues and I are more concerned about the heavy workload to be done in so little time than bickering on gender-related issues. If you are in the ward,... Read More |

Why is microbiology important in nursingMicrobiology is a very important topic for nurses to study. This is because our infectious diseases are caused by microbes. This was discovered in the late nineteenth century and is defined in the “Germ Theory of Disease”. In a few cases, even cancer is associated with particular infec... Read More |

As a nurse, have you ever cried over a patientYou bet I have. The 19 year old that we told would be okay, when we knew he wouldn't last the night ( auto accident with a crushed chest)And tears of joy. The 6 month old whose teen mother dipped in boiling water because he kept crying, The 85 year old who kept telling his wife “Don't leave me”,... Read More |

What are some surprising facts about newborn babies1. Babies are Born with the Skill to Swim:Newborns usually hold their breathing when underwater, and even throw about with their arms and legs.Your baby can go swimming as soon as you desire, but if you are preparing on getting her yourself, wait till after your six-week analysis. It is necessa... Read More |

What makes nurses cringe1. Seeing a diabetic check blood sugar without washing site prior2. Giving oneself insulin or other injectable medication as above3. Kids smoking4. Smoking adults5. &nbs... Read More |
What does it feel like to experience a heart attack?I was doing me first mow of the back yard for 2017. I’d mowed the front and was about halfway through the back yard. Over about five minutes I became unusually tired, breathless and started to sweat a LOT. I stopped mowing and thought about how out of shape I’d become. I started the mower again... Read More |

What's the most catastrophic mistake made by a nurse at a hospital?I was 17 years old and my mother was in the hospital and her concerns of not being able to breathe well were being dismissed by the nursing staff. They said “You are just getting yourself worked up”. We insisted she was having trouble breathing and needed some medication to help her breathe. The... Read More |

Global Nursing 2019Larix International Nursing ConferencesLarix International is a group of ranking publishers and organizer’s for scientific conferences around the globe nesting well-known Doctors, Engineers, Scientists, and Industrialists. Larix is a self-functioning, independent organization wholly focused on... Read More |

